A stomach for innovation?
A Q&A with CEOs of Evelo Biosciences and Abdul Latif Jameel Health on their exciting new adventure
In January 2021, Abdul Latif Jameel Health announced its first commercial partnership for therapeutic medicines with US-based Evelo Biosciences, a clinical stage biotechnology company developing a new modality of orally delivered medicines.
Under the recently announced agreement, the two companies will collaborate to develop and commercialize Evelo’s lead inflammation product and COVID-19 therapeutic medicine candidate, EDP1815 across the Middle East, Turkey and Africa.

We caught up with Dr. Simba Gill, Chief Executive Officer of Evelo Biosciences, and Akram Bouchenaki, CEO of Abdul Latif Jameel Health, to discuss the science; the ambitions behind the new collaboration and the opportunities they hope to explore.
What is novel about the medicines that Evelo are developing?

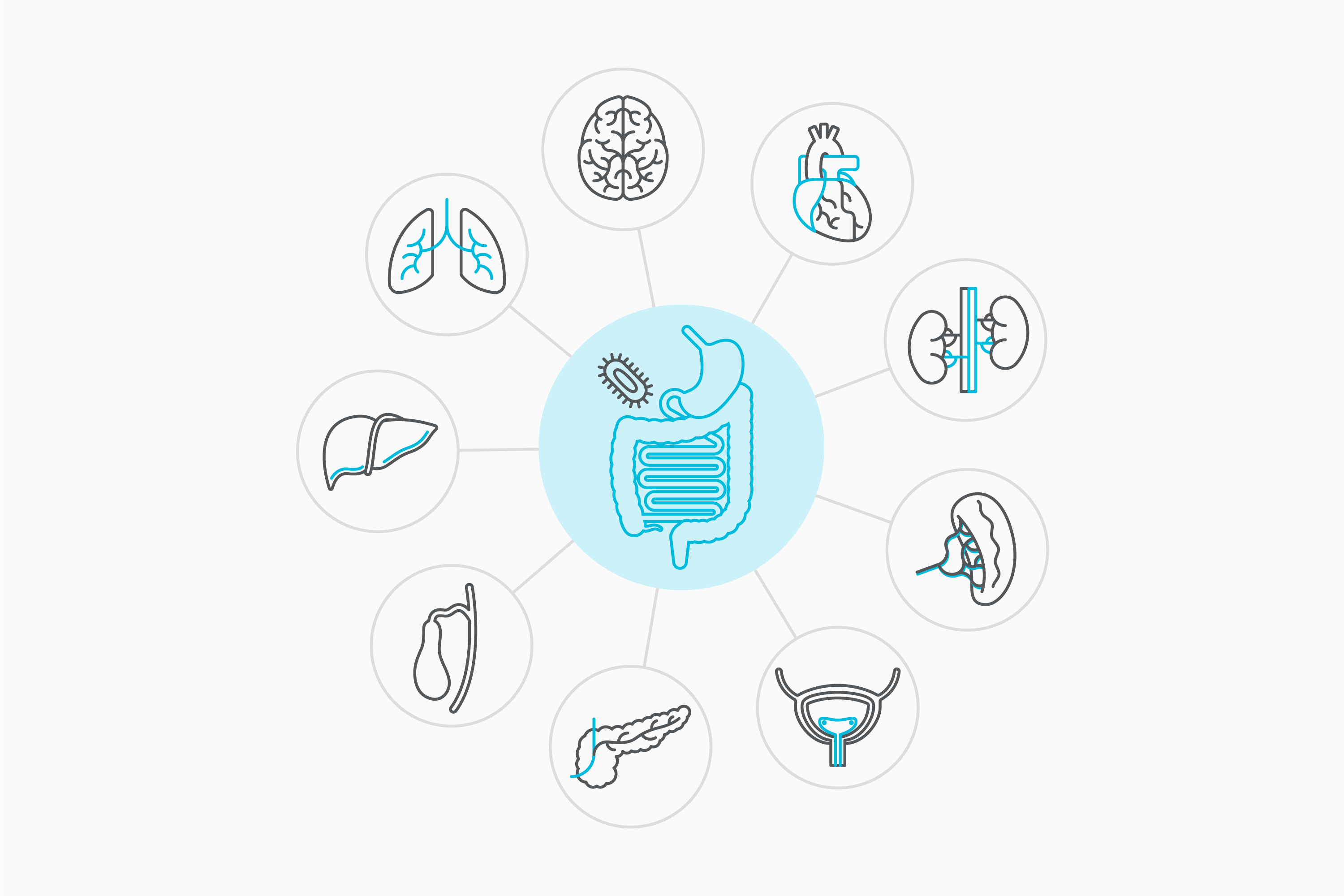
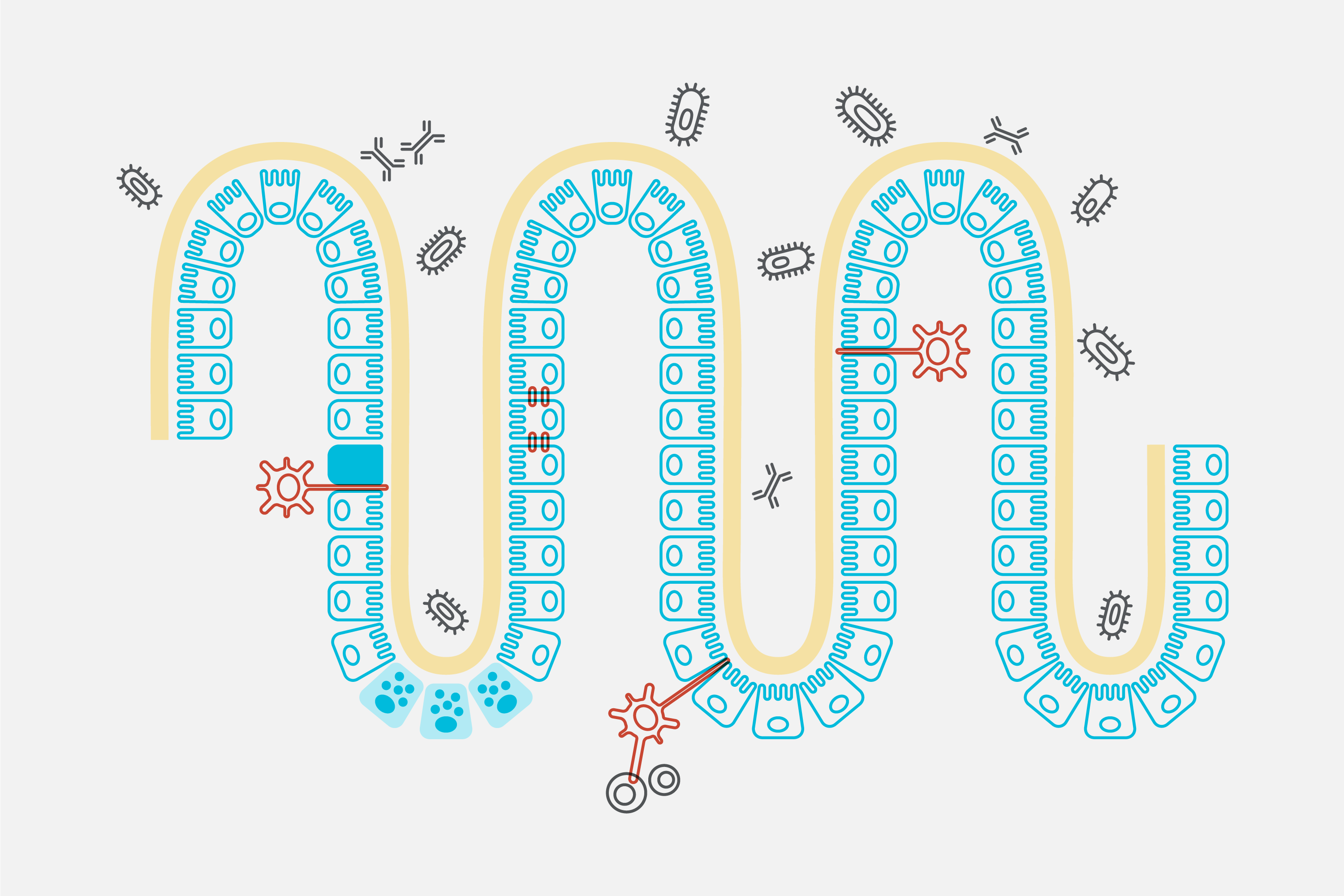
SG: At Evelo, our focus is on a newly uncovered area of biology. It’s recently been established that the small intestine governs biology throughout the body. It senses microbes in the external environment and, depending on what it senses, is able to modulate biology throughout the body. It’s a very simple and fundamental part of our biology that we’ve not known about before.
This opens up the possibility of delivering pharmacological agents to the relevant cells in the small intestine, which direct them to modulate a particular response throughout the body.
What that translates to, which we’ve now validated clinically, is the ability to create a completely new profile of medicine that is effective and orally delivered.
One that can be developed, manufactured, and distributed on an affordable basis, and is room-temperature stable. That may sound obvious – a drug that is effective, safe, affordable, and room-temperature stable – but actually, very few biotech medicines have that profile.
What benefits does this deliver?
SG: If you can develop medicines with this profile, it delivers two big benefits. First, it makes it much easier to treat what we call ‘the majority world’, that is, the soon-to-be 9 billion people who live outside the wealthy nations of the World.
Currently, only a very small percentage of patients who would benefit from biotech drugs are actually able to access them. A lot of this is down to price. For example, a typical inflammatory drug in the US is priced at around US$ 60,000 per course of therapy per patient. That’s obviously limiting for the majority world.
Another limitation is the stability of the drug. To be viable in the majority world, the drug needs to be room-temperature stable, so it can be administered in a wide range of settings. But as we’ve seen with the COVID-19 vaccine roll-out and issues around storage, that is hard to achieve.
 Safety and tolerability are also essential considerations. Most drugs have side-effects and tolerability issues because of what we call ‘off-target engagement’. For example, in a simplistic sense, chemotherapy attacks and destroys any cell that is rapidly dividing. But that not only includes cancer cells, it includes other cells that rapidly divide, too, such as our hair cells, which is why cancer patients often lose their hair. This is true for almost every drug at different levels, but it can be avoided with our novel medicines.
Safety and tolerability are also essential considerations. Most drugs have side-effects and tolerability issues because of what we call ‘off-target engagement’. For example, in a simplistic sense, chemotherapy attacks and destroys any cell that is rapidly dividing. But that not only includes cancer cells, it includes other cells that rapidly divide, too, such as our hair cells, which is why cancer patients often lose their hair. This is true for almost every drug at different levels, but it can be avoided with our novel medicines.
The data that we’ve recently reported has shown that we can drive meaningful clinical effect in inflammation with a drug that is well-tolerated and room-temperature stable. We’ve also now scaled-up our manufacturing to ensure that we can develop and manufacture on a very affordable basis, which makes it much easier to treat that majority world, including obviously Africa, Asia, the Middle East, and other high growth markets.
The second big benefit of the profile of our potential medicines is that it makes it easier to treat disease as early as possible. Most major chronic diseases aren’t effectively treated early on. Inflammation and the dysregulation of the immune system are central drivers of most major chronic diseases across all sorts of different areas, including multiple sclerosis, Parkinson’s, dementia, Alzheimer’s, asthma, food allergy, dermatitis, psoriasis, Crohn’s disease… the list goes on. But we don’t treat them early because we don’t have that profile of orally delivered, safe, affordable and effective medicines which ideally are room-temperature stable.
This new profile of drugs we are developing enables us to intervene at all stages of disease, shifting healthcare to something that is much more about preventing the development of serious disease, allowing us to live much longer and healthier lives as we go forward. The initial focus is on inflammation, but it has much broader application than that. It will also impact metabolic diseases and conditions, like diabetes, because diabetes and obesity are all linked to inflammation as core drivers.
How do you hope to make these drugs affordable?
 SG: There are two elements to this. One is that the way we manufacture our products, and the way we can distribute them, is much more cost-effective than classic biotech drugs.
SG: There are two elements to this. One is that the way we manufacture our products, and the way we can distribute them, is much more cost-effective than classic biotech drugs.
For example, cell therapies, which are very much in vogue right now, are extremely complex to manufacture, and because of that, they’ll always be very expensive.
I reject the idea that innovation needs to be associated with high price. Our products are, in the first instance, specific forms of bacteria. The manufacturing process is still very complex, but once you’ve figured it out, you can scale it up extremely cost-effectively.
Plus, the fact it is room-temperature stable means it is easier and cheaper to ship it around the world as you don’t need special refrigerated facilities. And it is easier and cheaper to administer if you can give someone a tablet, rather than needing to inject them.
The second point is the business model. Traditionally, the biotech industry was predicated on a high price, low volume model, like the luxury goods market.
But if you are brave and can flip that around to focus on high volumes and lower prices, it opens up the potential to help billions of people.
To do that, you need to create a mindset that prioritizes the value in treating billions of people.
Arguably, it’s the most important thing we can do to improve humanity right now, more so even than the environment. Protecting our environment is obviously critically important, however damage to the environment is not going to drive negative impact at a global scale for probably about a generation, whereas right now there are billions of people whose lives could be drastically improved with better access to effective medicines. And if you’re selling to billions of people, the fact the unit price is lower is not an issue, commercially. We’re talking about high-volume innovation, and that’s completely new as a concept.
Simba, what attracted you to the collaboration with Abdul Latif Jameel Health?
SG: The fit between our two organizations is just fantastic. You need courage and vision to try and do things that have never been done before, and Abdul Latif Jameel Health has plenty of both.
 It is very much aligned with our mission to impact global healthcare with novel medicines that can be distributed to a large population at an affordable price.
It is very much aligned with our mission to impact global healthcare with novel medicines that can be distributed to a large population at an affordable price.
The classic biotech multinational will always prioritize the US, and then probably Europe and Japan in that order, depending on the company, and then as an afterthought, you have all the other geographies. But Abdul Latif Jameel Health does not think like that. There is an entrepreneurial spirit and an understanding of the world that one will not get, certainly in an America-based multinational, or even in a Europe-based multinational.
The strong sense we had was that Abdul Latif Jameel Health really understands these markets, these ‘majority world’ geographies, and understands how to act entrepreneurially to create something significant in these territories.
Akram, what was it about Evelo that fitted into the mission of Abdul Latif Jameel Health?
 AB: There are a number of factors that made the Evelo partnership such a strong one for us. First is the science. The innovation that Evelo has developed, and the excellent research, the clinical work, and the speed at which they have developed their therapies is really unique, especially given the current circumstances with the pandemic.
AB: There are a number of factors that made the Evelo partnership such a strong one for us. First is the science. The innovation that Evelo has developed, and the excellent research, the clinical work, and the speed at which they have developed their therapies is really unique, especially given the current circumstances with the pandemic.
I truly believe it will unlock a whole new field of medicine for the future. Second is the vision to provide access to innovative medicines to a largely under-served parts of the world, which intersects perfectly with our own ambitions. It is at the core of the Abdul Latif Jameel Health mission, and now we are seeing it in motion.
The third element is my relationship with Simba and the deep respect and admiration I have for what he has achieved, not only at Evelo, but from his earliest days in the research labs in the UK and his role at Maxygen, which was a pioneer in the field of biotech, through to more recent involvement at a number of biotech innovators, like Gilead Sciences, where we first met over 20 years ago. These are the three elements that make the deal such an important one for me.
Could you explain the nature of the collaboration and what you hope to achieve?

CEO Abdul Latif Jameel Health
AB: The collaboration is very classic in the sense that Evelo is the innovator company, and we are building the channel to commercialize and make that innovation available. So, that’s the simple high-level picture.
In detail, the interesting angle is that Evelo and Abdul Latif Jameel Health are jointly going to build the market for this compound. We need to educate and inform the medical community about the importance of these new medicines. So, there is an important piece of work that needs to happen, and that we will be doing this together and jointly with Evelo.
We are also supporting Evelo as an investor, as well as a commercial partner, which reinforces the alignment and the collaboration between the two groups.
SG: Evelo’s core focus is on innovation and translating scientific innovation to clinical results. We’ll take the lead on that. Abdul Latif Jameel Health has a strong presence in all the relevant markets across Africa and the Middle East, and so they will lead on building out the infrastructure and the capabilities, and everything that’s associated with opening up those territories.
There are about 1.7 billion people in the geographies we’ll be targeting, maybe more.
Our goal at Evelo is to get our medicines to people in need on a global basis, so this really matters to us.
What do you see as the biggest challenges on that journey?
AB: There are some macro challenges in the region in that the usual innovations take longer to reach our target geographies. On average, it takes seven years for a US-approved drug to reach the Middle East, for example. It’s even longer, if ever, to get to some parts of Africa. So, the number one challenge is to make sure we overcome the hurdles to accelerate access. Part of that is about ensuring the regulatory issues are managed efficiently. Then, it will be about building the infrastructure to support the launch and educating the healthcare community about the new drug.
Of course, the context and the specific challenges will vary for different countries, but that’s one of the key strengths of Abdul Latif Jameel Health – the ability to adapt and adjust to local contexts and address the issues as they arise.
Your lead product candidate is EDP1815. What is it and what does it do?
SG: EDP1815 is a pharmaceutical preparation of a naturally occurring single strain of commensal bacteria. Commensal bacteria are bacteria that live within us naturally that are completely harmless. Our platform has allowed us to identify EDP1815 as a specific microbe which, when you deliver it in a tablet or a capsule, properly formulated, the pre-clinical and clinical data shows that we’re able to resolve inflammation associated with multiple inflammatory diseases, as well as COVID-19.
Another huge opportunity is in treating otherwise poorly treated forms of cancer. We’ve shown pre-clinically and clinically that by activating the immune system, we can improve its ability to detect, attack and kill cancer cells throughout the body.
How can EDP1815 help in the fight against COVID-19?
SG: It’s generally recognized that progression to serious COVID-19 is driven by the body’s overreaction to the virus, its hyper-inflammatory response – the ‘cytokine storm’ you often hear referenced in news media. This has resulted in a number of companies looking at anti-inflammatory drugs as potential treatments for COVID-19. They have had some effect, but it’s been limited. In the best case, they reduce mortality for very late-stage COVID-19 patients but are less effective in earlier stages of the illness. So why not? The suggestion is that although anti-inflammatories can stop the inflammatory response that causes the progression of COVID-19, they are so potent that they also shut down the ability of our own immune system to fight the virus.
What we’ve demonstrated with EDP1815 is a ‘goldilocks effect’ – it reduces the disease-progressing inflammatory response but does not shut down our own natural ability to fight the virus.
If we demonstrate success, it really opens up the ability to use EDP1815 for the broad community-based treatment of COVID-19 from a very early stage. Something you can take orally at home before going into the hospital. You can’t do that with an injectable drug, which brings us back to the core thesis of Evelo – an effective, affordable, orally taken medicine that is stable at room temperature.
In the future, coronavirus will become endemic, not to mention new variants. Even with vaccines, many people across the globe will still catch COVID-19 every year, so there is still going to be a huge need for medicines to fight it.
So, with this first partnership signed, what is next for Abdul Latif Jameel Health?
AB: Our goal is to continue to build relationships with other partners as strong as Evelo in terms of quality and impact on the therapeutic space.
The other focus is the work we’re doing on the device and diagnostic space. Screening and diagnosing early, and broadly, are the main catalysts that lead to healthier societies. So, we’re active in that direction as well, looking for innovative, impactful solutions in the field of diagnostics and screening.
I hope we’ll be soon in a position to announce more deals that can take us further along this path towards a future where access to good quality healthcare does not just depend on how wealthy you are.





 1x
1x


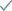 Added to press kit
Added to press kit


